logo
logo
- 바이넥스
- 바이오의약품 CDMO
- 합성의약품
- 투자정보
- 윤리경영
- 채용정보
- CDMO 사업개요
- CDO
- CMO
- Quailty
Your True and Reliable CDMO Partner, BINEX
BINEX is a leading biologics Contract Development and Manufacturing Organization (CDMO) with accumulated experience and expertise. With an enhanced quality management system and diverse facilities, we offer ideal solutions for successful biopharmaceutical development.
We provide comprehensive CDMO services, catering to diverse needs from early-stage development (including cell line development, process development, and analytical method development) to GMP production and regulatory support. With our commitment to taking responsibility for every step in the production process, even the smallest ones, we dedicate our efforts to achieving our client’s goal together.
Core Competency
- Abundant

Track Record - Flexible

Manufacturing Facility - Client

Oriented CDMO - One-stop
CDMO Service
Abundant Track Record
BINEX has been supporting the development and production of biopharmaceuticals for over 140 domestic and international clients.We have successfully completed inspections by regulatory authorities in various countries and continuously monitor and enhance cGMP and quality systems through periodic inspections by regulatory agencies and audits from multinational pharmaceutical companies.
- Clients140+
- GMP Batches1200+
- Inspection & Audits40+
- IND/BLA Approval95+
Flexible Manufacturing Facility
BINEX offers production services ranging from small to large scale, tailored to clients’ development strategies and required quantities, spanning from small to large scale. Utilizing various types of bioreactors such as mammalian cell bioreactors and microbial fermenters, we offer a one-stop service covering the entire process of biopharmaceutical production, from the production of drug substances to the final formulation of drug product(liquid vials, lyophilized vials, pre-filled syringes).


| Plant | Classification | Suite | Scale (type) * | Train (W.V.) * |
|---|---|---|---|---|
| Songdo | Mammalian | Suite 1 | 200 L (SUB) | 200 L |
| 1,000 L (SUB) | 200 L - 1,000 L | |||
| Suite 2 | 500 L (STS) | 500 L | ||
| Suite 3 | 1,000 L (STS) | 200 L - 1,000 L | ||
| Suite 4 | 1,000 L (STS) | 200 L - 1,000 L | ||
| Microbial | Suite 5 | 180 L (STS) | 180 L | |
| Suite 6 | 500 L (STS) | 500 L |
| Plant | Type | Available Size | Capacity |
|---|---|---|---|
| Songdo | Liquid Vial | 2 – 50 mL | 10,000 vials/batch |
| Lyophilized Vial | 2 – 50 mL | 5,000 vials/batch | |
| Pre-Filled Syringe | 1 – 2.25 mL | 6,000 vials/batch |
| Plant | Classification | Suite | Scale (type) * | Train (W.V.) * |
|---|---|---|---|---|
| Osong | Mammalian | Suite 7 | 1,000 L (STS) | 1,000 L |
| 1,000 L (STS) | 200 L - 1,000 L | |||
| Suite 8 | 5,000 L (STS) | 1,000 L - 5,000 L |
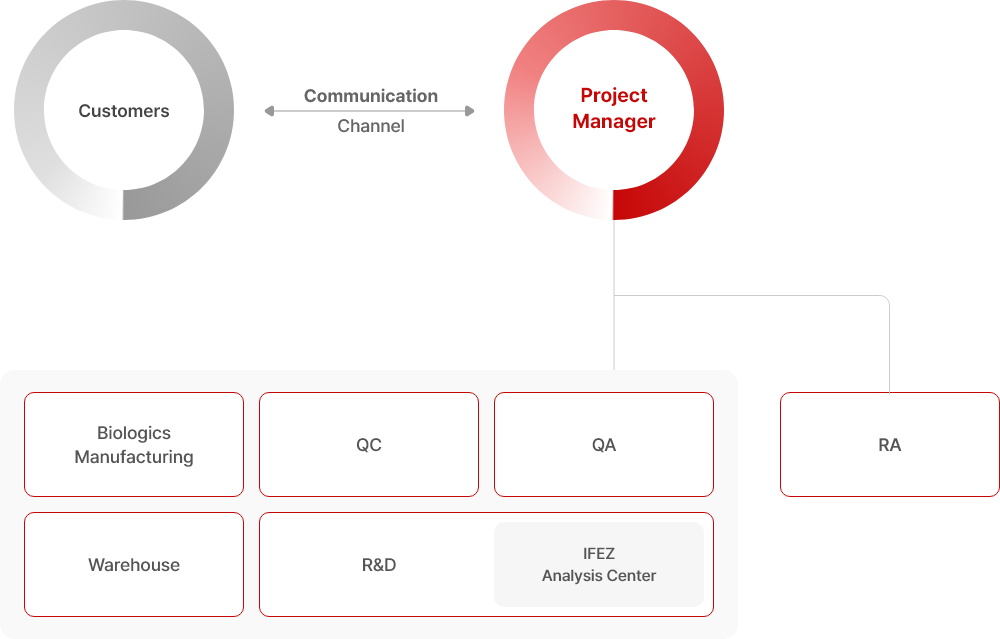
Client Oriented CDMO
BINEX maintains ongoing communication with clients throughout the entire project. Each project is accompanied by a dedicated Project Manager (PM) who acts as a bridge between relative departments and the client, ensuring completion of planned schedules and mission. We achieve efficient and systematic task execution under PM supervision.
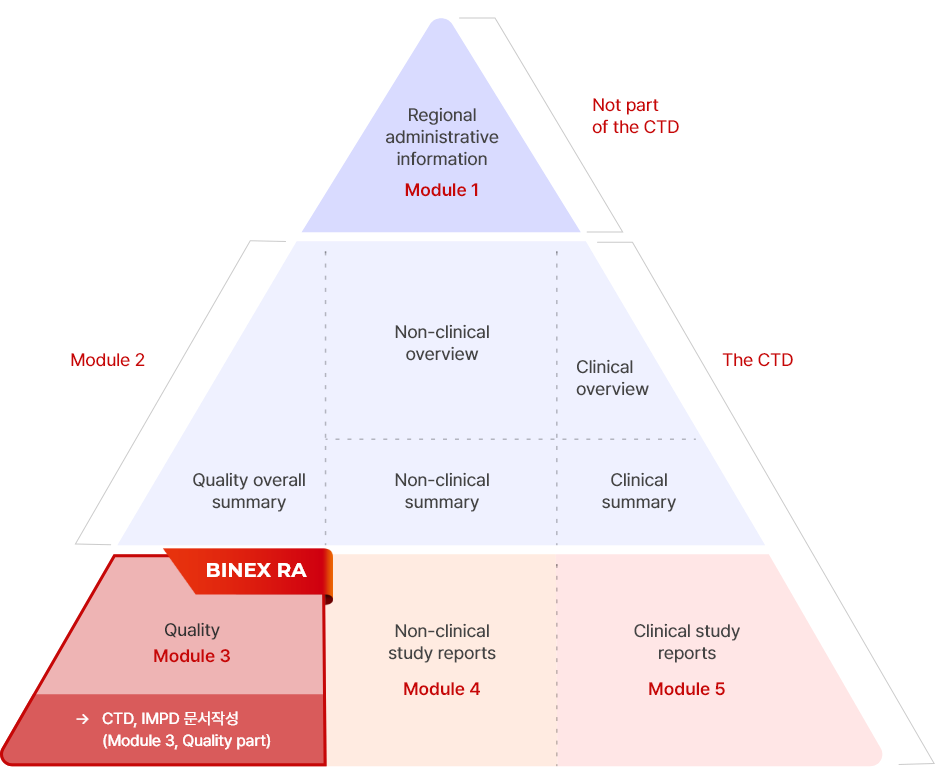
One Stop CDMO Service
From cell line development to regulatory document support, BINEX provides one-stop service over the entire biopharmaceutical development process.
- More Details

- Cell Line Development
- Optimized CLD services for client's product through comparative application of various expression systems
- Cell lines establishment with assured productivity, viability, monoclonality, and sterility
- More Details

- Process/Analytical Method Development
- Process development platform optimized for large-scale production
- Reliable analytical methods development tailored to the characteristics of the project
- More Details

- GMP Manufacturing(DS/DP)
- Total 13,180 L capacity of manufacturing facilities designed and prepared to meet the needs of clients’ diverse process and scales
- Industry-leading expertise in mammalian cell cultivation and microbial fermentation across multiple modalities
- More Details

- QC Testing/RA Support
- QC testing that meets the requirements and standards of global regulatory agencies such as FDA, EMA, and PMDA
- QA professionals with experience in inspections by domestic and international regulatory authorities






