logo
KORENG
logo
바이오의약품 CDMO
- 바이넥스
- 바이오의약품 CDMO
- 합성의약품
- 투자정보
- 윤리경영
- 채용정보
CMO
- CDMO 사업개요
- CDO
- CMO
- Quailty
Contract Manufacturing Services
BINEX takes on responsibility of being a manufacturing partner for clients, aiding in the commercialization and global expansion of their product.
The process of developing pharmaceuticals can be complex and unpredictable, with various stages bringing both diversity and volatility. BINEX is committed to ensuring the consistent production of high-quality pharmaceuticals by rigorously managing our quality system. Drawing on years of production experience and technical expertise, we offer flexible services tailored to client’s needs.
Drug Substance
- Capabilities that enable manufacturing from clinical to commercial product
- Mammalian cell: 200 ~ 5,000L
- Microbial: 180, 500L
- Extensive production experience with various product types
- Providing cell banking services (MCB/WCB) within a GMP facilities
- GMP 환경 내 cell banking 제조(MCB/WCB) 서비스 제공

| Classification | Suite | Scale (type) | Train (W.V.) * |
|---|---|---|---|
| Mammalian | Suite 1 | 200 L (SUB) | 200 L |
| 1,000 L (SUB) | 200 L - 1,000 L | ||
| Suite 2 | 500 L (STS) | 500 L | |
| Suite 3 | 1,000 L (STS) | 200 L - 1,000 L | |
| Suite 4 | 1,000 L (STS) | 200 L - 1,000 L | |
| Suite 5 | 1,000 L (STS) | 1,000 L | |
| 1,000 L (STS) | 200 L - 1,000 L | ||
| Suite 6 | 5,000 L (STS) | 1,000 L - 5,000 L | |
| Microbial | Suite 7 | 180 L (STS) | 180 L |
| Suite 8 | 500 L (STS) | 500 L |
* W.V.: Working Volume
- Monoclonal
Antibody - Bispecific
Antibody - Fc-fusion
Protein - Recombinant
Protein - pDNA
(gene therapy) - Recombinant
Vaccine
(DNA vaccine) - (Exosome,
Viral Vector,
etc.)
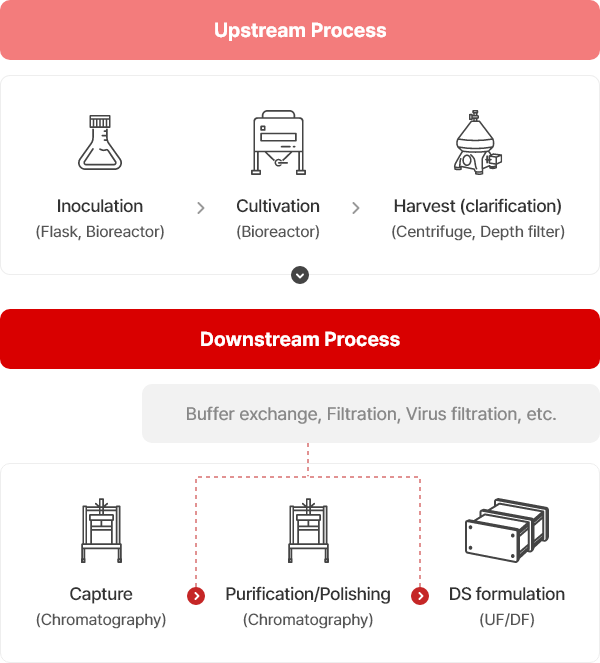
Process Flow

Buffer exchange, Filtration, Virus filtration, etc.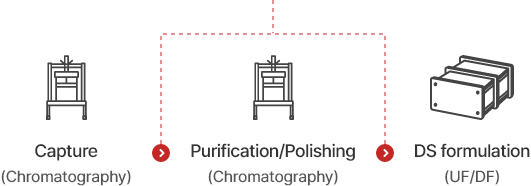

Upstream ProcessDownstream Process